Hugo Bisio Sabaris, winner of an ERC Proof of Concept 2025 award
12 September 2025
A novel dialogue between proteins and RNA at the heart of bacterial sporulation, Clémentine Delan-Forino
8 October 2025
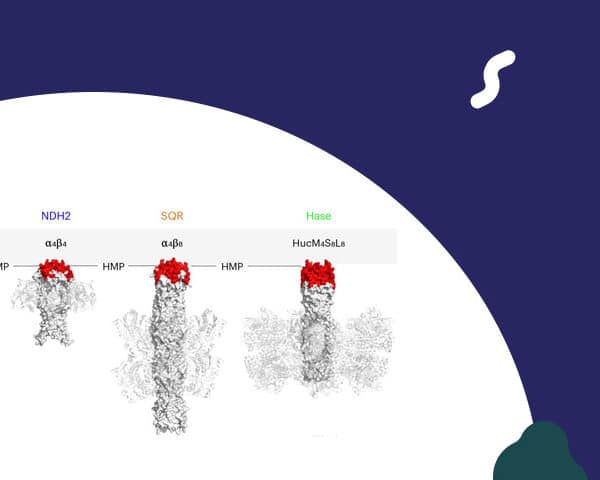
Anne Walburger (Axel Magalon‘s team, LCB Marseille) has published in Nature Structural & Molecular Biology the discovery of a novel module involved in the transfer of quinones, the essential molecules that act as electron shuttles in cells.
By studying the bacterial enzyme ForCE (formate dehydrogenase) using crystallography and cryo-electron microscopy, the researchers revealed an original architecture, consisting of four catalytic subunits arranged around a central membrane scaffold.
At the heart of this device is a hitherto unknown conserved domain known as HMP (Helical Membrane Plugin). This module is essential for linking formate oxidation to the aerobic respiratory chain in Bacillus subtilis.
Bioinformatics analyses reveal that the HMP domain is associated with numerous other oxidoreductases, suggesting that it represents a universal link in electron transfer between catalytic centers and the quinone pool.